A quiet beast is crawling.
Antibiotic Resistance (AR) is the silent pandemic of the 21st century. The extensive use of antibiotics leads to dangerous pathogens acquiring immunity to the drugs designed to kill them.
The longest war of humankind.
Health systems around the world work hard to save lives. Sadly, every day multiple bacterial strains develop antibiotic resistance, and the consequences are deathly. Experts expect this trend to increase in the following years, resulting in a potential worldwide crisis.
The more we fight, the stronger it becomes.
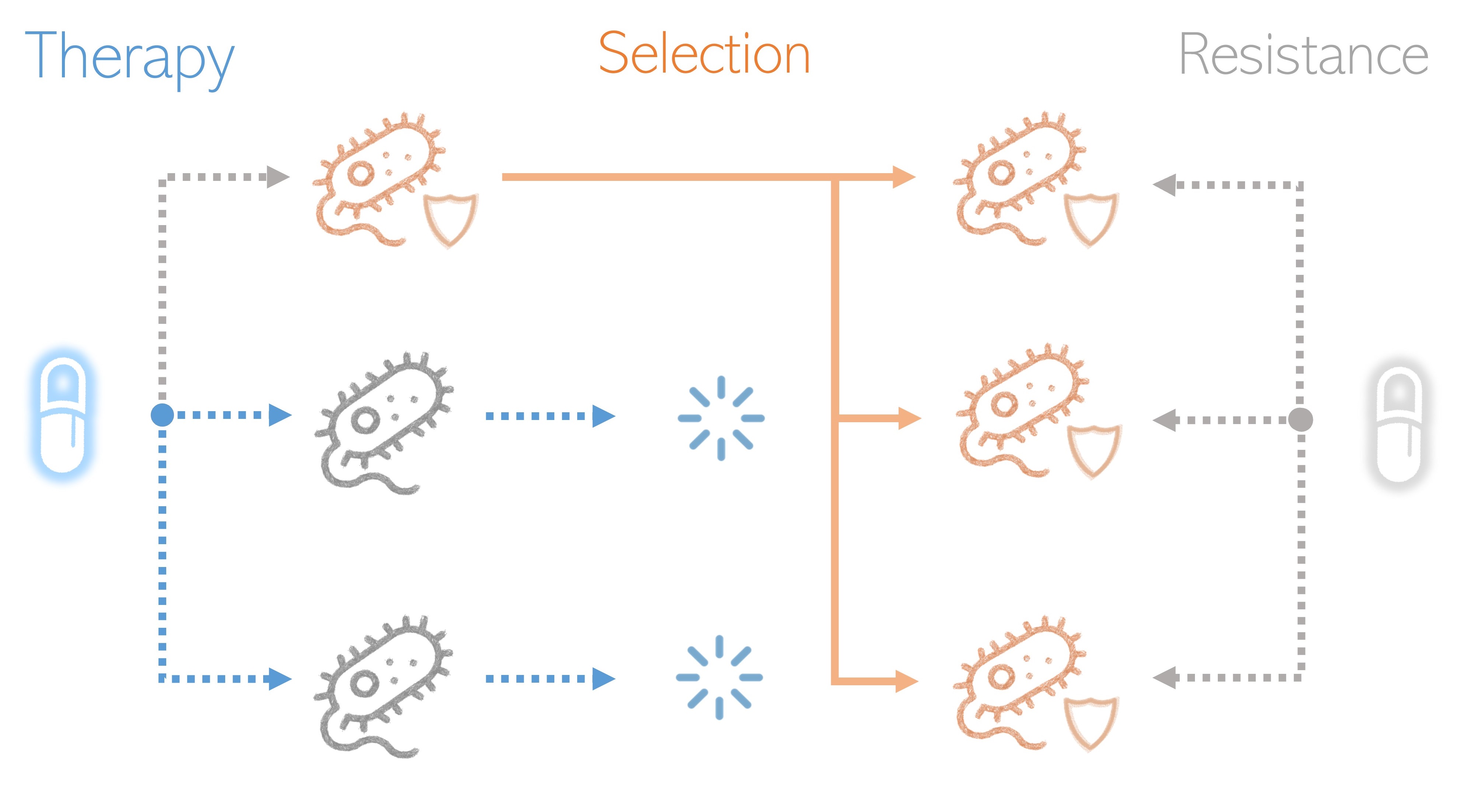
In its effort to eliminate dangerous pathogens, medicine has unintentionally contributed to the acceleration of a powerful force: evolution. By removing those microorganisms vulnerable to current drugs, ecological space becomes free for resistant individuals to develop and expand. With time, this process results in whole populations of resistant pathogens, which render our defenses useless.
A highly complex problem.
AR is not a simple phenomenon: it depends on an intricate group of factors, including genetics, proteomics, genomics, environmental conditions, pharmacodynamics, patient physiology, etc. Furthermore, bacteria present powerful mechanisms to share biological information between individuals, strains, and even species. To tackle this problem, all elements must be integrated and understood, as they all are crucial for AR development and spread.
A multidisciplinary team.
Such a great challenge moved 11 Human Biology and Biomedical Engineering undergrads to team up and discuss an action plan. This seed-like idea finally became the Antibiotic Resistance Inference Array Project, which we call ARIA.
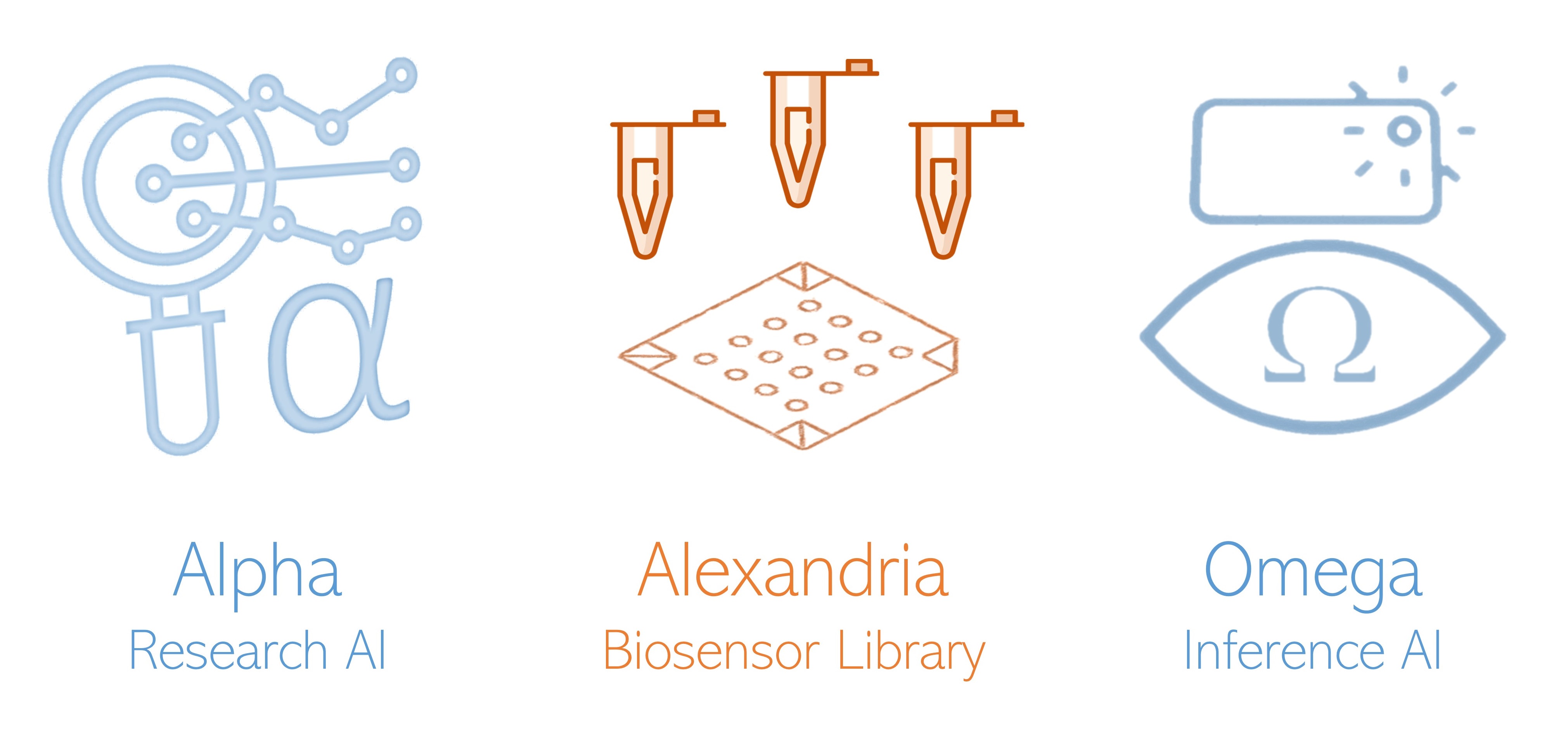
Our proposal: Synbio + AI.
ARIA is founded upon two promising fields: synthetic biology and artificial intelligence. Our goal is to develop the proof-of-concept for an open, accessible, and efficient system to fight back this dangerous enemy. This system seeks to provide clinicians with helpful and accurate information that assists them when saving patients. To do so, we are working on three main modules, each of them materializing one of the three steps in our strategy: Alpha, Alexandria, and Omega.
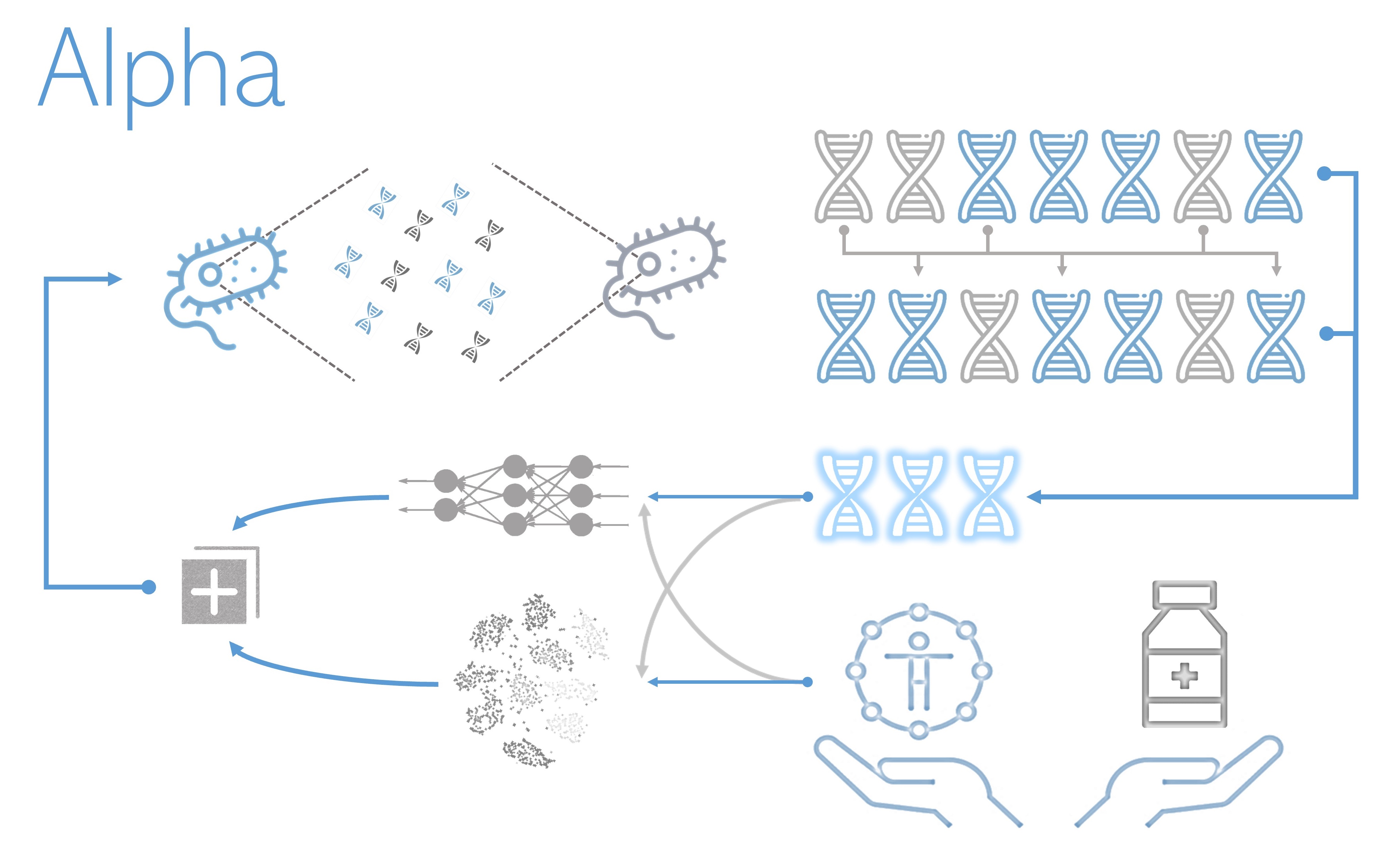
1) Find, research, and characterize AR threat profiles.
Alpha is an artificial intelligence system intended to find, analyze and understand the inner mechanisms of resistance, virulence, and gene transfer. To do so, it feeds on vast amounts of data. Such data includes genomes/genes/plasmids (both from resistant and susceptible bacteria), drug features, and patient statistics. A combination of deep learning, advanced algorithms, massive parallelization, and direct connection to standardized databases make Alpha able to condense all this data into models composed of AR hallmarks.
2) Create an array of self-growable biosensors to detect the profiles.
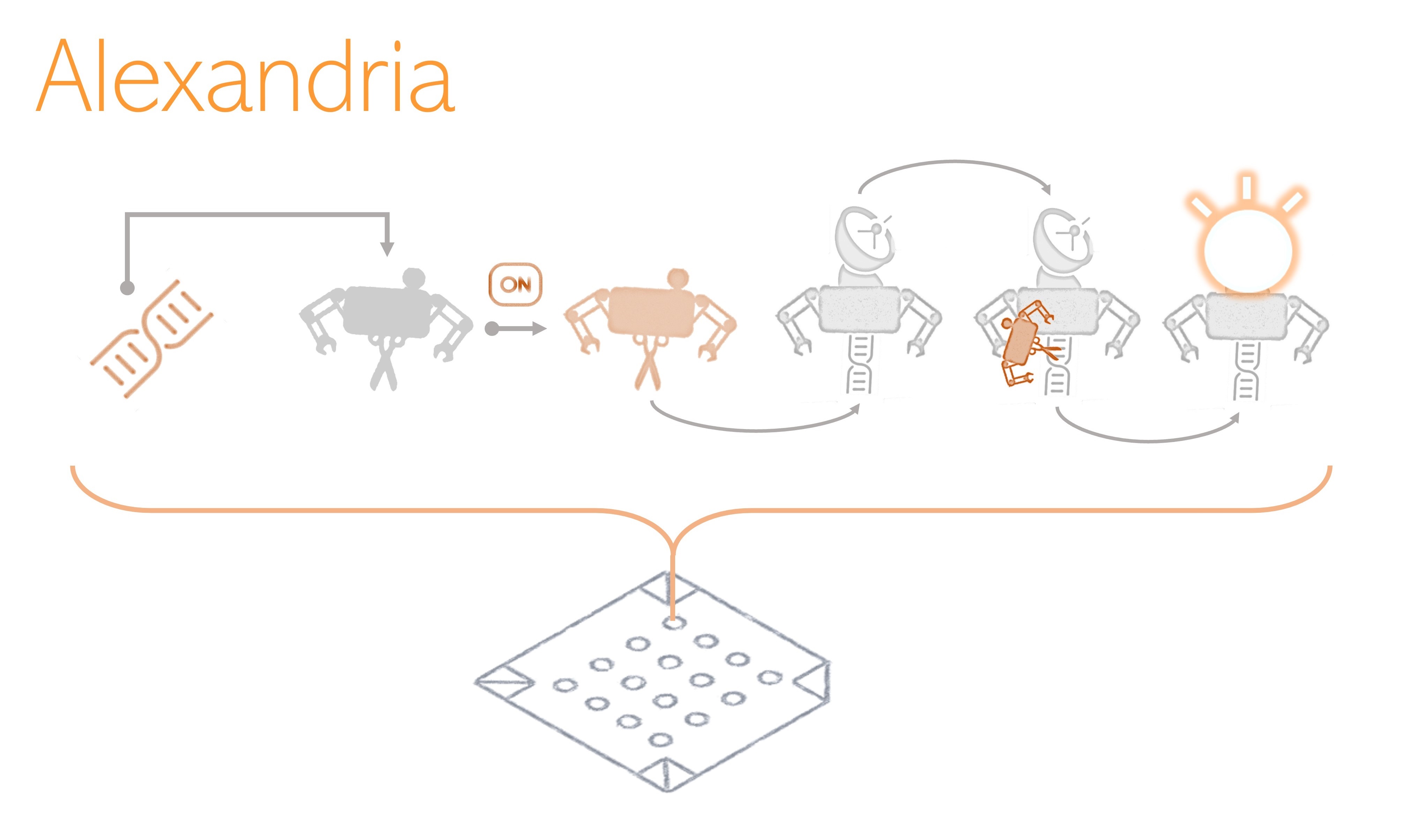
With well-defined biological AR hallmarks, we work on CRISPR-based biosensors that release an optical signal (fluorescence) when interacting with them. Furthermore, we use synthetic biology to make these constructs self-growable, so we can conveniently produce a complete library of biosensors, which we call Alexandria. We deploy this library in a paper-based array. In that way, we build a device to which we can add pathogenic samples containing genetic material: the result is a specific activation pattern describing the pathogen's resistance configuration, based on our AR hallmarks.
3) Identify the profiles using just an internet connection.
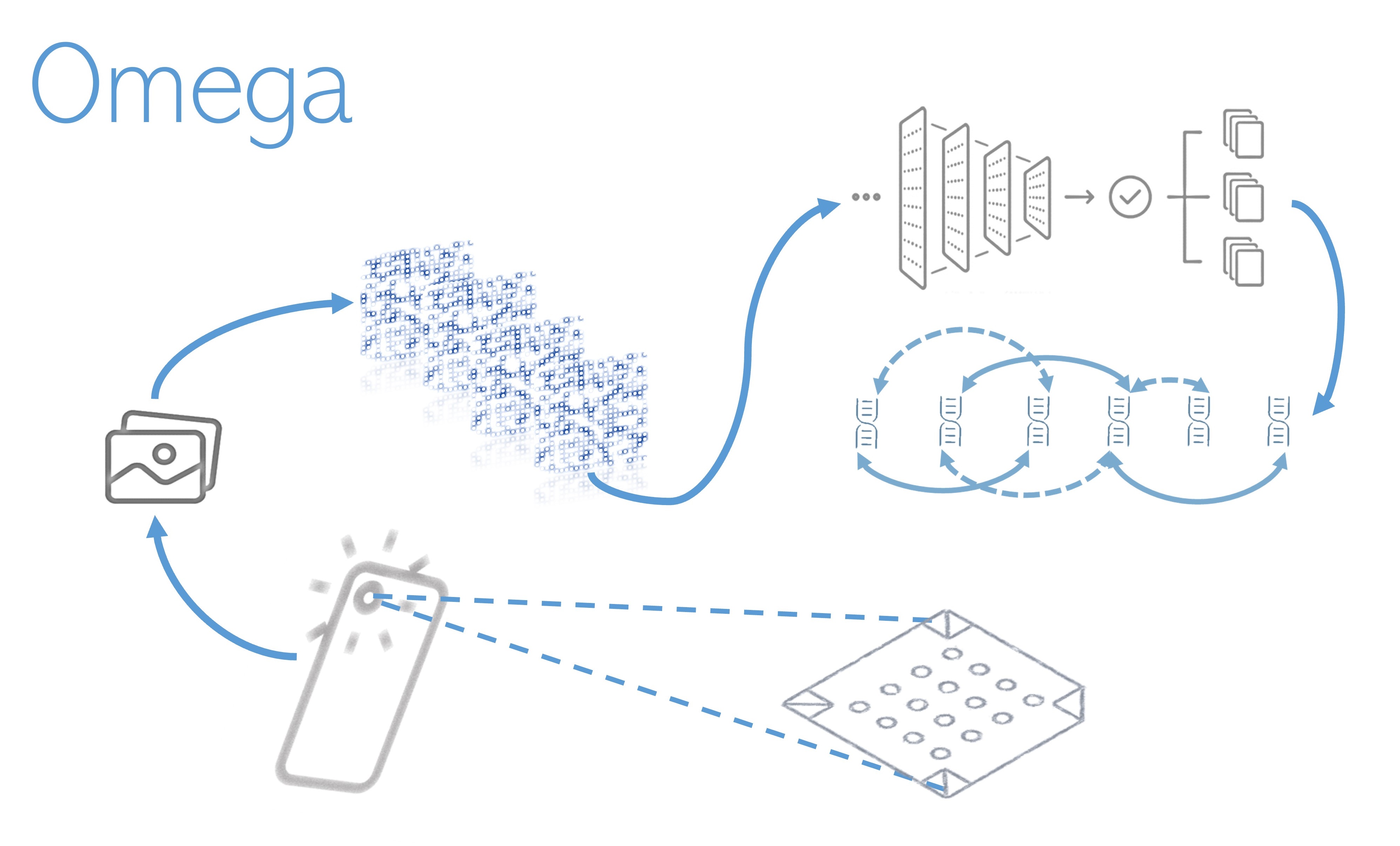
We develop a cross-platform app to capture an image of the array’s fluorescence pattern. The app converts it into an embedding and sends it to Omega together with patient-specific parameters. Thus, our second artificial intelligence system deeply analyzes the expression signals found in the sample array and its relation with the patient's condition. The goal is to find the connection between the pathogen studied and Alpha’s functional models. Doing this, Omega reports the potential weaknesses of the pathogenic biological system and recommends the most efficient countermeasures to neutralize it.
Our multidisciplinary team
11 talented Biomedical Engineering and Human Biology undergrad students. 6 experienced instructors.

Project gallery
Discover with a few images how do we work.

Donate
Put your two cents in a socially compromised project. It will not be possible without your help.